Why Calibration and Validation Aren’t Just Paperwork
Think of calibration like checking your kitchen scale before baking a cake. If it’s off by even a few grams, your cake might not rise right. Now imagine that scale is measuring the dosage of a life-saving drug. A tiny error isn’t just a bad batch-it’s a patient at risk. That’s why equipment calibration and validation aren’t optional checklists in manufacturing. They’re the backbone of quality, safety, and compliance.
Every piece of equipment that measures, tests, or controls a process-whether it’s a micrometer, a pH meter, or a robotic arm-must be calibrated regularly. But calibration alone isn’t enough. You also need validation to prove the whole system works as intended in real-world conditions. These two processes work together. One ensures accuracy. The other ensures reliability.
What Calibration Actually Means (And What It Doesn’t)
Calibration isn’t just adjusting a device. It’s a documented process that compares your equipment’s readings to a known standard-something traceable back to the International System of Units (SI). For example, a temperature sensor might be tested against a NIST-traceable reference thermometer in a controlled lab. The difference between what your device reads and what the standard says is the error. That error gets recorded, and if it’s outside acceptable limits, the device gets adjusted or replaced.
ISO 13485:2016, the global standard for medical device quality management, says calibration must happen at specified intervals or before use. But here’s the catch: there’s no universal schedule. A high-precision micrometer in an aerospace plant might need calibration every 3 months. A basic thermometer in a food packaging line could last 18-24 months. The key is risk. If a measurement error could harm a patient or cause a product recall, you calibrate more often.
And don’t forget the environment. NIST Technical Note 1900 found that 57.8% of out-of-tolerance failures happen when temperature or humidity swings too much. If your lab isn’t climate-controlled, even a perfectly calibrated device can drift fast. That’s why many medical device manufacturers now use IoT sensors to monitor environmental conditions in real time-and only calibrate when needed, not just because the calendar says so.
Validation: Proving the System Works, Not Just the Tool
Calibration tells you your scale is accurate. Validation tells you the whole baking process-mixing, heating, cooling-produces the right cake every time. In manufacturing, validation means proving your equipment does what it’s supposed to do under actual operating conditions.
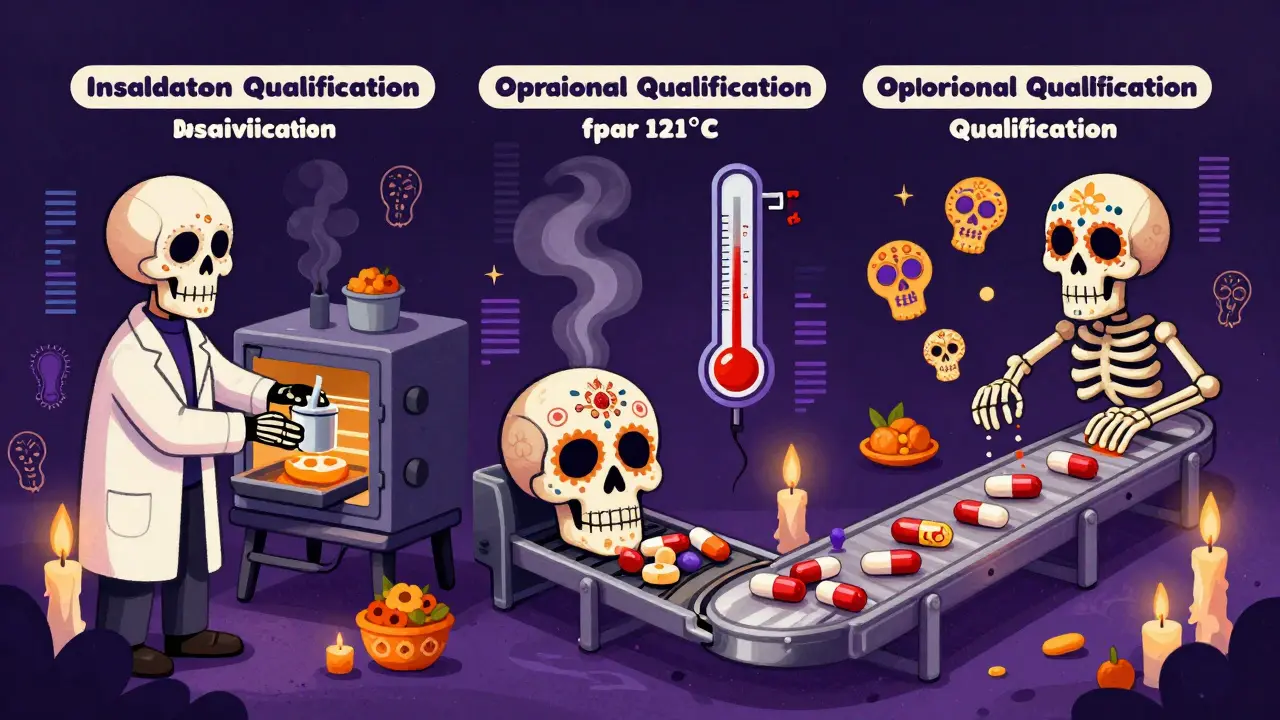
This is broken into three phases:
- Installation Qualification (IQ): Did you install the equipment correctly? Are all parts present? Are the manuals and software versions correct?
- Operational Qualification (OQ): Does it work across its full range? Test it at minimum, maximum, and normal settings. For example, a sterilizer must reach and hold 121°C for 15 minutes at full load.
- Performance Qualification (PQ): Does it consistently produce quality output? Run multiple batches under normal conditions and check the results.
Validation is expensive-between $25,000 and $500,000 per system, depending on complexity. But the cost of a failed validation? A recall, a regulatory shutdown, or worse. FDA warning letters in 2023 showed 37.2% cited inadequate calibration or validation procedures. That’s not a fine. That’s a business stoppage.
How Standards Differ-and Why It Matters
Not all regulations are the same. ISO 13485:2016 requires every calibration to be traceable to SI units. No exceptions. CLIA, which governs clinical labs, allows some point-of-care devices (like glucose meters) to skip full calibration if they pass daily verification checks. That’s a 23.5% reduction in workload for waived tests, according to CMS 2023 data.
ISO 9001:2015 lets you extend calibration intervals if you have data showing the equipment stays stable. But ISO 13485 doesn’t allow that unless you’ve formally validated the extension. This is why medical device companies often follow the stricter rule-even if they’re not required to.
And then there’s geography. The EU’s MDR 2017/745 requires traceability to BIPM (International Bureau of Weights and Measures). The FDA accepts NIST traceability. If you sell globally, you might need two sets of calibration certificates. That adds 18.7% to compliance costs, according to a 2023 McKinsey study of 45 medical device firms.
Real-World Problems and How Smart Companies Fix Them
Here’s what goes wrong-and how to avoid it:
- Calibrating too often: A Reddit thread from April 2024 had 63 users saying manufacturer-recommended intervals are too frequent. One biomedical engineer extended electronic scale calibration from quarterly to biannually after 18 months of stable data-saving $18,500 a year.
- Calibrating too rarely: Another user reported pH meters in a high-humidity lab needed monthly calibration, even though the manual said every 6 months. Environmental conditions matter more than the manual.
- Documentation overload: Small manufacturers spend 15.2 hours a week just managing calibration records, per a 2024 FDA survey. That’s not quality control-that’s paperwork prison.
The fix? Calibration management software. Tools like GageList or Trescal automate certificate generation, send reminders, store records digitally, and pull data from IoT sensors. Companies using these systems cut audit prep time by 63.2%, from 84 hours to 31 hours per week.
And then there’s AI. Pfizer ran a pilot using AI to predict when equipment would drift, scheduling calibrations only when needed. They cut calibration costs by 31.7%. But NIST warns: 44.2% of automated systems fail to properly document the chain of custody for reference standards. If you can’t prove your standard was never tampered with, your calibration is worthless.

What You Need to Get Started
Building a compliant program takes time-usually 6 to 9 months. Here’s the path:
- Inventory everything: List every measuring device. Assign a unique ID to each. No exceptions.
- Classify by risk: High-risk devices (e.g., those measuring drug concentration) get frequent calibration. Low-risk (e.g., basic thermometers) get less.
- Set intervals using Method 5: Combine manufacturer recommendations, historical performance data, and risk assessment. Companies using this method reduced non-conformances by 27.4%.
- Control the environment: Temperature and humidity matter. If you’re in a warehouse with no climate control, invest in ISO Class 5 environmental chambers. They cost $85,000-$120,000, but prevent 41.3% of calibration failures in semiconductor and medical device settings.
- Train your team: NCSL International’s MET-101 course covers metrology basics. ASQ’s Certified Calibration Technician (CCT) credential is held by over 14,000 professionals globally-and those with it earn 22.5% more.
The Future: Continuous Monitoring, Not Calendar Checks
Calibration is changing. The old model-calibrate every 6 months, document it, file it away-is fading. The new model is continuous verification.
ISO published Amendment 1 to ISO 13485:2016 in March 2024, requiring calibration of AI/ML-based systems through ongoing validation. That means algorithms that predict defects or adjust machine settings must be monitored for drift, just like a sensor.
The FDA’s 2024 Calibration Modernization Initiative mandates all electronic records for Class II and III device manufacturers by December 31, 2026. That’s 14.2 million paper records eliminated annually.
NIST’s roadmap for quantum-based calibration promises 100x more accuracy by 2030. Imagine a pressure sensor so precise it never drifts. Calibration intervals could stretch from months to years.
But there’s a bottleneck: technician shortages. ILAC’s 2024 survey found 83.6% of calibration labs report staffing gaps. Forty-seven accredited labs closed in 2023 because they couldn’t hire qualified people. That’s why investing in automation and training isn’t optional-it’s survival.
Final Thought: It’s Not About Compliance. It’s About Confidence.
Calibration and validation aren’t about passing an audit. They’re about knowing your product is safe, your patients are protected, and your team can sleep at night. The data doesn’t lie: companies that treat these processes as strategic, not administrative, see fewer recalls, faster audits, and higher customer trust.
Start small. Pick one high-risk device. Document its calibration. Validate its use. Build from there. The goal isn’t perfection-it’s control. And control, in manufacturing, is everything.


Lily Steele
January 31, 2026 AT 18:28Blair Kelly
February 1, 2026 AT 14:36Amy Insalaco
February 2, 2026 AT 01:17Marc Bains
February 3, 2026 AT 16:23Beth Beltway
February 5, 2026 AT 12:52Darren Gormley
February 6, 2026 AT 21:06Kathleen Riley
February 7, 2026 AT 00:41Eliana Botelho
February 8, 2026 AT 08:39Rohit Kumar
February 9, 2026 AT 22:51Katie and Nathan Milburn
February 11, 2026 AT 11:09